Challenges in U.S. Vaccine Distribution
For months, the Trump administration focused on the dazzling goal of a Covid-19 vaccine. But once the first candidate was approved, federal officials took a hands-off approach, leaving overwhelmed states to manage the vaccine rollout. American Oversight is investigating this lack of federal support and the disparate plans that emerged as a result.

In November 2020, then Health and Human Services Secretary Alex Azar said that 40 million Covid-19 vaccine doses would be available in the United States by the end of the year. Operation Warp Speed officials later amended the goal to 20 million distributed doses. But by the final day of 2020, two weeks after vaccinations began, only around 14 million doses had been distributed. Fewer than 3 million people had been inoculated.
At the same time, new, more contagious coronavirus variants began to spread, making vaccination efforts even more crucial. To reach widespread immunity by July 2021, the U.S. would need to vaccinate more than 2 million people every day. But the initial rollout, marred by an inefficient system, wasted doses, and racial disparities, fell far short of this goal.
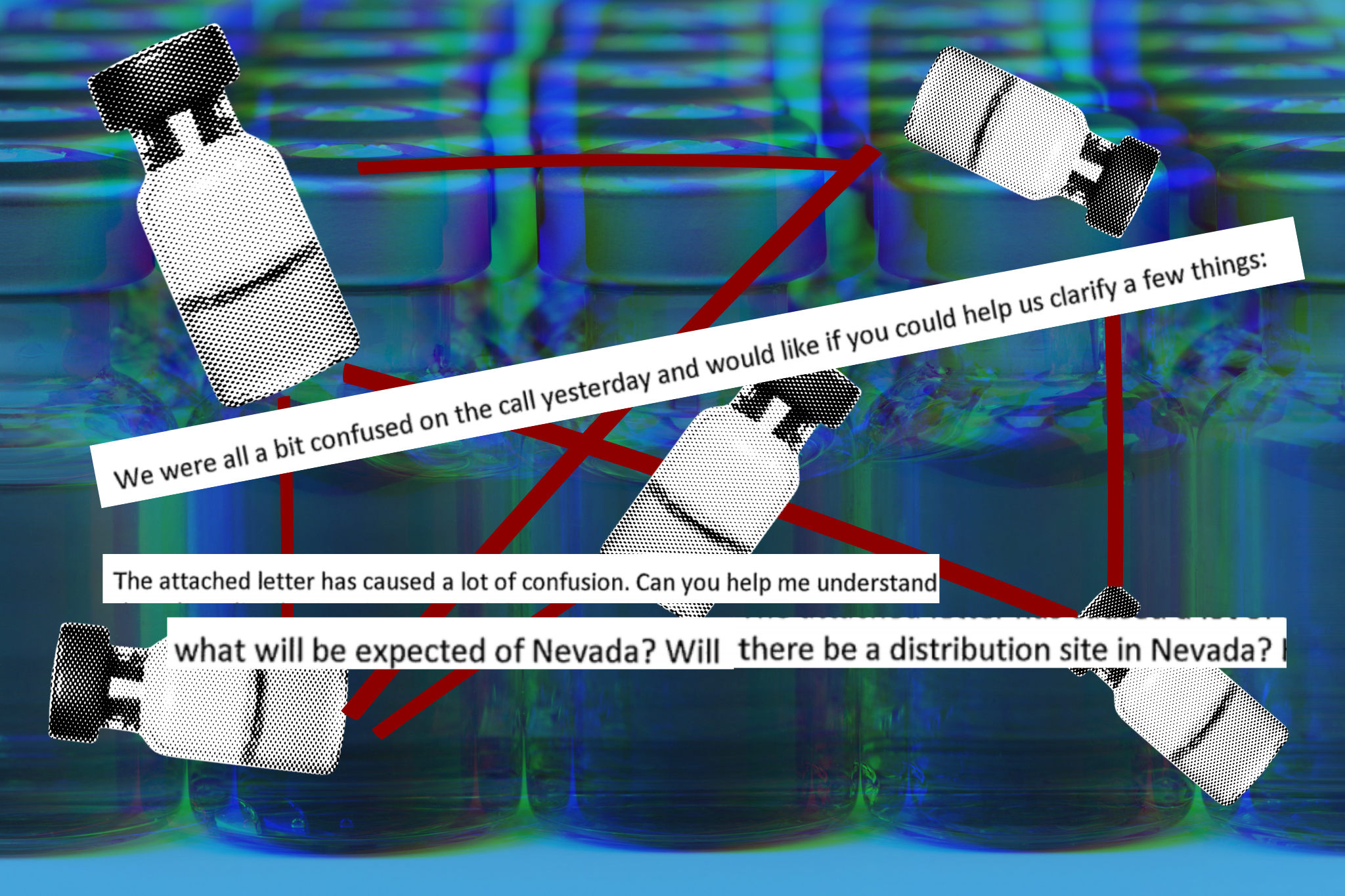
In the first two months, state officials struggled to communicate with federal counterparts, making it difficult to plan logistics like storing vaccines, making appointments, and scheduling inoculations. In December, state health departments were informed that they’d be receiving 20 percent to 40 percent fewer vaccine doses than they had expected, due to federal officials not adjusting for quality-control processes. In January, states expected an increase in vaccine supply after Azar announced that the government would release doses that had been held in reserve. Governors across the country expressed outrage when the Washington Post reported that no reserve existed — Operation Warp Speed had stopped stockpiling second doses of the Pfizer-BioNTech vaccine in December.
While struggling to coordinate with the federal government, states have also faced difficulty managing the millions of people who want vaccines. After vaccine eligibility was expanded beyond the first group of health care workers, registrations filled up within minutes, frustrating those who, despite being eligible, remained unable to acquire protection. Limited manufacturing capacity meant that these supply gaps wouldn’t be filled until April 2021.
And as states reported vaccine shortages in January 2021, federal officials were attempting to determine the whereabouts of more than 20 million doses, many of which officials feared could be sitting unused in warehouses. Since most Covid-19 vaccines were placed in units that could only store doses for 30 days, millions could go to waste if not used within the appropriate time.
Amid the confusion, minority populations have been left behind. In early February, just 5 percent of vaccine doses had gone to Black Americans and only 11 percent to Latinos, despite Covid-19’s disproportionate impact on these groups. Two months after the first Covid-19 vaccine was approved, millions of essential workers were still waiting for their inoculations, even though they often have the highest risk of exposure and are disproportionately people of color. Tracking vaccinations in communities of color was also difficult because most states were not publicly reporting racial data on vaccinations.
American Oversight is investigating the federal government’s plans for vaccine distribution. We’re also investigating state plans and have filed requests with officials in Arizona, Oklahoma, Nevada, and Wisconsin seeking communications about vaccine distribution plans. We’ve also filed public records requests with the Florida Division of Emergency Management and the state’s Department of Health seeking communications regarding problems with vaccine access.