The Rush for a Covid-19 Vaccine
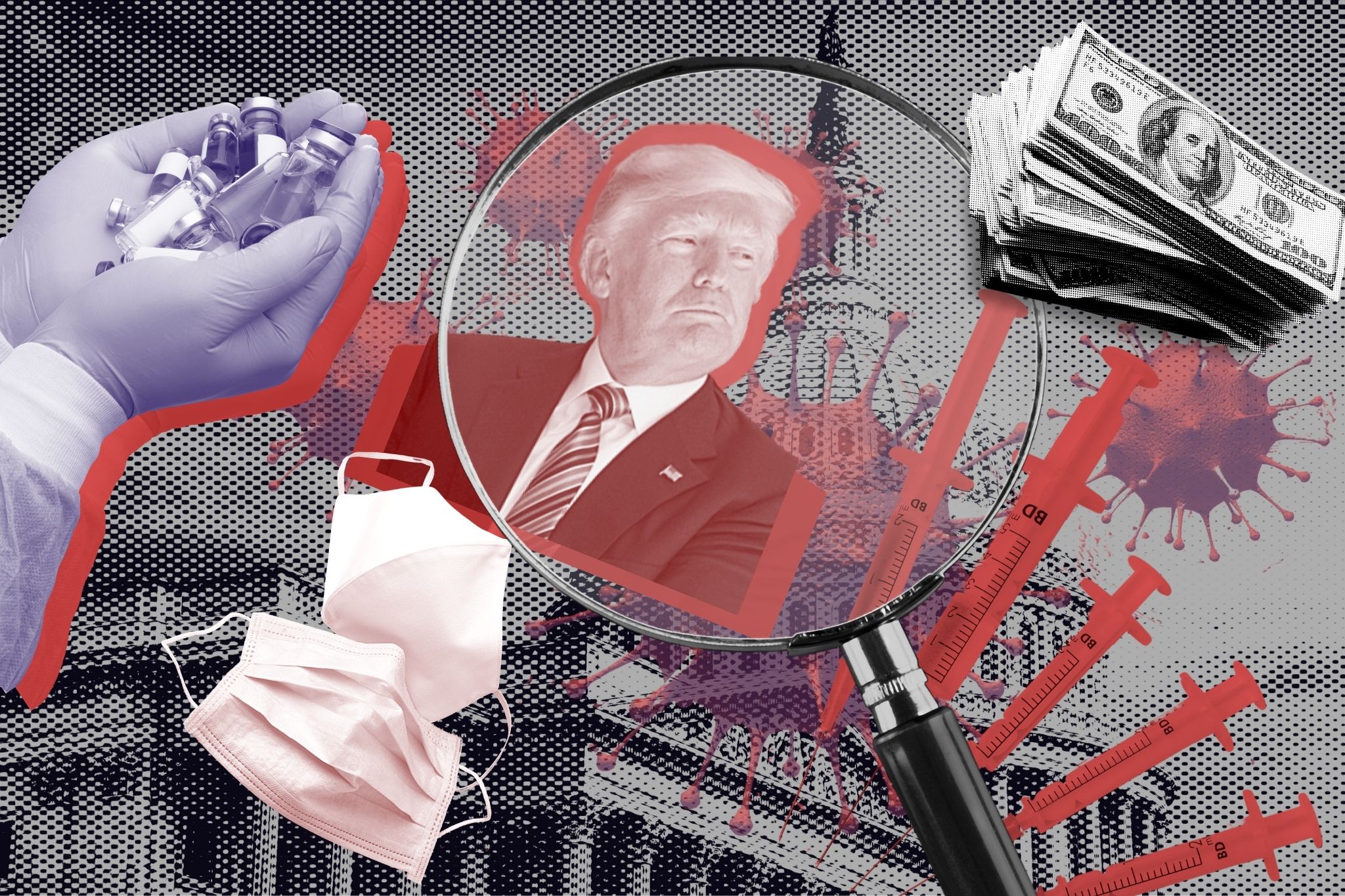
The pursuit of a coronavirus vaccine began with a hurried timeline, nakedly political motivations, and a lack of transparency that morphed into problems of disorganization and poor communication in the vaccines' distribution. American Oversight is investigating the key players and influences involved in the development and distribution of a vaccine.

On Sept. 7, then President Donald Trump told reporters, “We could have a vaccine soon, maybe even before a very special day. You know what day I’m talking about.” Although the first Covid-19 vaccine was granted emergency approval more than a month after Election Day, Trump’s remarks encapsulated his administration’s approach to the vaccine: an approach that prioritized speed over effectiveness and politics over public health, and was repeatedly unable to deliver on its grand promises.
In the early days of the coronavirus pandemic, the Trump administration launched Operation Warp Speed with an initial goal to have a vaccine ready in October 2020. The initiative funneled billions of dollars into promising vaccine candidates, supporting the research, manufacturing, and eventual distribution of these vaccines, with full payment contingent on companies meeting specific timing milestones.
In May 2020, Trump announced that Dr. Moncef Slaoui would be the initiative’s chief adviser, despite his glaring financial conflicts of interest. After Sen. Elizabeth Warren called him out for holding more than $12 million in stock options for biotechnology company Moderna, Slaoui sold his stake and resigned from the company’s board. But he maintained millions of dollars of shares in his former company, GlaxoSmithKline, which was a contender in the vaccine race and was supported by more than $2 billion in taxpayer money. And an investigation by the House found that he also had undisclosed holdings in Lonza Group, a biotechnology company that has a contract with Moderna to manufacture its coronavirus vaccine.
In August, as multiple vaccine candidates began to enter phase three trials, the Centers for Disease Control and Prevention notified state officials to prepare to distribute a coronavirus vaccine as early as late October, and Director Robert Redfield asked governors to prepare vaccine distribution sites by Nov. 1. A month later, the administration released a vaccine distribution plan that promised a free vaccine and called for states to submit distribution plans to the CDC by Oct. 16. Officials also rushed a new and untested system to track vaccines.
At the same time, leaders of science agencies cautioned against expecting a vaccine so soon and faced Trump’s anger for it. After Centers for Disease Control Director Robert Redfield said that a vaccine likely wouldn’t be widely available until months into 2021, Trump attacked him publicly. Trump also dismissed and threatened to deny White House approval to Food and Drug Administration plans for stricter guidelines of vaccine authorization, which included more rigorous criteria for clinical trials and a recommendation that a committee of independent experts review the data. Previously, Trump claimed that the “deep state” at the FDA was attempting to delay the vaccine process until after the election.
Despite the administration’s rush to get states ready, vaccine distribution measures fell woefully flat after the Pfizer-BioNTech vaccine became available in December 2020. Federal officials took a hands-off approach, leaving the details of vaccine distribution to overwhelmed state health departments. These state officials struggled with unclear communications from HHS officials, who even alluded in January 2021 to a reserve of vaccine doses that did not exist.
American Oversight is investigating the management of the vaccine process and has filed multiple requests for agency and White House communications about coronavirus vaccines. We’ve also filed requests seeking contracts and communications with leading vaccine developers, including Pfizer, Moderna, and AstraZeneca. Finally, we’ve filed requests with state officials seeking communications with federal officials about coronavirus vaccine plans.